Osmium Tetroxide
Other Names:
Osmium(VIII) oxide
General Information:
Structure:

CAS Number: 20816-12-0
Molecular Weight: 254.23 g/mol
Appearance: Pale yellow solid (sublimes at room temperature)
Melting Point: 39.5-41 C
Boiling Point: 130 C
Osmium tetroxide (OsO4) is a highly toxic reagent that is typically used to make vicinal diols from alkenes. It is soluble in many organic solvents, only moderately soluble in water.
Common Uses:
Reagent for the conversion of alkenes to diols
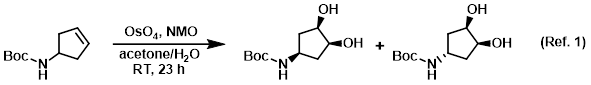
Procedure excerpt:
. . . the SM (1.94 g, 10.58 mmol) in a mixture of acetone (63 mL) and H2O (7.8 mL) at RT was added NMO (2.48 g, 21.16 mmol). After 2 min, OsO4 (4% in H2O, 3.37 mL) was . . .
Reagent for the conversion of alkenes to aldehydes (via oxidative cleavage)
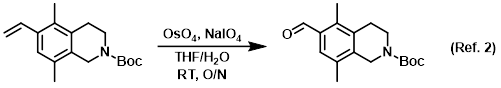
Procedure excerpt:
The SM (1.03 g, 3.58 mmol), NaIO4 (2.34 g, 10.9 mmol), OsO4 (2.5 wt% in t-BuOH, 1.0 mL), THF (12.4 mL), and H2O (2.4 mL) were combined at RT. The reaction mixture . . .
Safety:
Osmium tetroxide is highly toxic and is made more dangerous by the fact that it is a solid that sublimes at room temperature. Extreme care should be taken when using osmium tetroxide. Inhalation of amounts below that point at which they can be detected by smell can be enough to lead to pulmonary edema, then death. Osmium tetroxide can stain human cornea leading to blindness.
References:
1) Patent Reference: WO2016001341, page 126, ![]() (9.1 MB)
(9.1 MB)
2) Patent Reference: WO2016014463, page 98, ![]() (6.7 MB)
(6.7 MB)
3) Wikipedia: Osmium tetroxide (link)
4) www.sigmaaldrich.com: Osmium tetroxide (link)
5) Fuchs, P. L.; Handbook of Reagents for Organic Synthesis, Catalytic Oxidation Reagents
